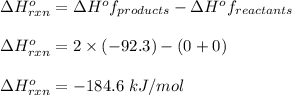Chemistry, 19.09.2020 01:01, emileehogan
Hydrogen gas, H2, reacts explosively with gaseous chlorine, Cl2, to form hydrogen chloride, HCl(g). What is the enthalpy change for the reaction of 2 mole of H2(g) with 2 mole of Cl2(g) if both the reactants and products are at standard state conditions? The standard enthalpy of formation of HCl(g) is −92.3 kJ/mol. H2(g)+Cl2(g)→2HCl(g)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 05:00, jjoyner
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2

Chemistry, 23.06.2019 16:50, alexisbaronetp85kek
How can a scientist assess whether a pure niobium (nb) sample is responsible for contaminating the lab with radioactivity? test the niobium sample to see whether it now contains other elements. test the niobium sample for the presence of niobium oxide compounds. heat the niobium, and see if the level of radioactivity in the lab increases. place the niobium under pressure, and see if the level of radioactivity in the lab increases.
Answers: 3

Chemistry, 23.06.2019 19:00, gabrielar80
Freya built a model of the earth, sun, and moon. freya can cause her model to move in three different ways. she can turn the crank to model how the earth revolves around the sun, she can spin the globe to model how the earth rotates on its axis, and she can push the moon with her fingers to model how the moon revolves around the earth. how could freya best use her model to demonstrate the cause of the day/night cycle on earth?
Answers: 1

Chemistry, 23.06.2019 22:00, chaparro0512
Acertain substance has a solubility of 12 grams in 100 grams of water at 20°c. this means that when 12 g of the substance are dissolved in 100 grams of water, the solution will be saturated when 12 g of the substance are dissolved in 100 grams of water, the solution will be dilute the substance will begin to dissolve when 12 grams are present in solution when 12 grams of the substance are stirred into a beaker with 100 g of water, it will begin settling at the bottom
Answers: 3
Do you know the correct answer?
Hydrogen gas, H2, reacts explosively with gaseous chlorine, Cl2, to form hydrogen chloride, HCl(g)....
Questions in other subjects:


Biology, 21.07.2019 23:20



Mathematics, 21.07.2019 23:20

Mathematics, 21.07.2019 23:20

History, 21.07.2019 23:20

Health, 21.07.2019 23:20


Arts, 21.07.2019 23:20


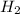 , reacts explosively with gaseous chlorine,
, reacts explosively with gaseous chlorine, 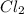 , to form hydrogen chloride, HCl(g).
, to form hydrogen chloride, HCl(g).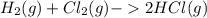
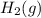 with 2 mole of
with 2 mole of 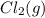 if both the reactants and products are at standard state conditions .
if both the reactants and products are at standard state conditions .