
Chemistry, 10.09.2020 03:01, CoreyHammond1517
When 229.0 J of energy is supplied as heat to 3.00 mol of Ar(g) at constant pressure the temperature of the sample increases by 2.55 K. Assuming that in the experiment the gas behaves as an ideal gas, calculate the molar heat capacities at constant volume and at constant pressure of Ar(g).

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, samantha9430
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 15:20, shanyeah
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 22.06.2019 15:30, sanchez7489
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1
Do you know the correct answer?
When 229.0 J of energy is supplied as heat to 3.00 mol of Ar(g) at constant pressure the temperature...
Questions in other subjects:











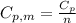
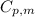 is the molar heat capacity at constant pressure
is the molar heat capacity at constant pressure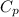 is the heat capacity at constant pressure
is the heat capacity at constant pressure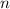 is the number of moles
is the number of moles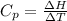
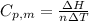
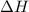 = 229.0 J
= 229.0 J = 2.55 K
= 2.55 K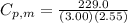
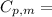 29.93 JK⁻¹mol⁻¹
29.93 JK⁻¹mol⁻¹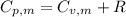
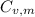 is the molar heat capacity at constant volume
is the molar heat capacity at constant volume 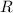 is the gas constant (
is the gas constant (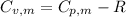
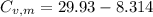
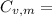 21.62 JK⁻¹mol⁻¹
21.62 JK⁻¹mol⁻¹



