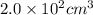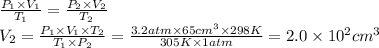An air bubble at the bottom of lake with temperature 7 0C and pressure of 3.2 atm has a radius of 2.5 cm. It rises to the surface where the temperature is 25 oC and atmospheric pressure is 1 atm. What is the volume of the bubble at the surface? Assume perfect gases. V=(4/3)πr3.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:00, Janznznz4012
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 23.06.2019 05:30, jalynholden07
Based on the formulas, select the compounds below that are covalent: kbr sif4 al2o3 co2 naco3 s7o2 pcl3 fe3n2 h2o s2f10
Answers: 3

Chemistry, 23.06.2019 07:00, jaydenboi604
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample?
Answers: 1

Chemistry, 23.06.2019 07:30, lucas2020197
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 2
Do you know the correct answer?
An air bubble at the bottom of lake with temperature 7 0C and pressure of 3.2 atm has a radius of 2....
Questions in other subjects:

Biology, 22.08.2019 00:30




Mathematics, 22.08.2019 00:30

Mathematics, 22.08.2019 00:30


Social Studies, 22.08.2019 00:30