
Chemistry, 09.09.2020 08:01, lwilliams28
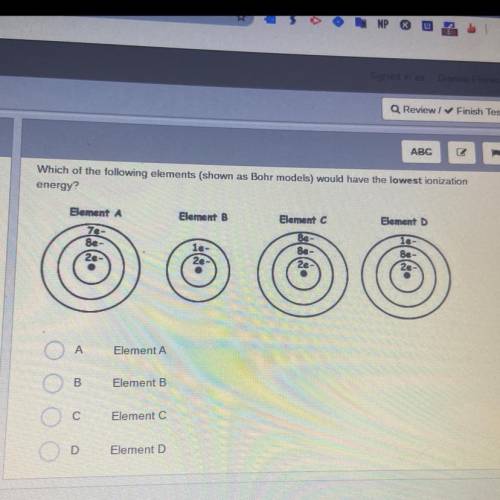
Which of the following elements (shown as Bohr models) would have the lowest ionization
energy?
Element A
Element B
Element a
Element D
ro
save
le-
7e-
8e-
2e
le-
Be
8e
2e
8e-
2e
Circuits
esign
.ce
riods of
device.
table
A
Element A
B
Element B
С
Element C
D
Element D

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, reeceslife481
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 04:00, breannaasmith1122
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 06:30, 91miketaylor
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2
Do you know the correct answer?
Which of the following elements (shown as Bohr models) would have the lowest ionization
energy?
Questions in other subjects:







Mathematics, 22.10.2019 16:50


Mathematics, 22.10.2019 16:50






