
Chemistry, 08.09.2020 14:01, awesomegrill
Part AGold-191 undergoes electron capture. Express your answer as a nuclear equation. Part BGold-201 decays to a mercury isotope. Express your answer as a nuclear equation. Part CGold-198 undergoes beta emission. Express your answer as a nuclear equation. Part DGold-188 decays by positron emission. Express your answer as a nuclear equation.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, sammiehammer
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2



Chemistry, 22.06.2019 21:30, Turtlelover05
How can the periodic table be used to predict the behavior of elements?
Answers: 1
Do you know the correct answer?
Part AGold-191 undergoes electron capture. Express your answer as a nuclear equation. Part BGold-201...
Questions in other subjects:

Mathematics, 16.10.2020 15:01

Physics, 16.10.2020 15:01

English, 16.10.2020 15:01


Spanish, 16.10.2020 15:01

Mathematics, 16.10.2020 15:01


Social Studies, 16.10.2020 15:01

Mathematics, 16.10.2020 15:01

English, 16.10.2020 15:01


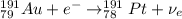
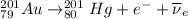
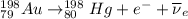
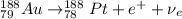
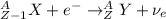
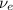 ) is emitted.
) is emitted. 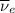 ) are emitted from the Au-201 nucleus and a neutron is converted to a proton (n-1 and Z+1; A remains constant).
) are emitted from the Au-201 nucleus and a neutron is converted to a proton (n-1 and Z+1; A remains constant). 



