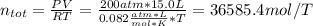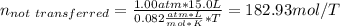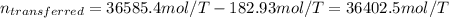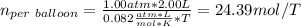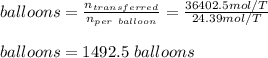Chemistry, 08.09.2020 14:01, luisgonz5050
A 15.0 L tank is filled with helium to a pressure of 2.00 * 102 atm. How many balloons (each 2.00 L) can be inflated to a pressure of 1.00 atm, assuming that the temperature remains constant and that the tank cannot be emptied below 1.00 atm?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:40, keiracoles
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 16:40, roderickhinton
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
Do you know the correct answer?
A 15.0 L tank is filled with helium to a pressure of 2.00 * 102 atm. How many balloons (each 2.00 L)...
Questions in other subjects:

Health, 07.11.2020 01:50


History, 07.11.2020 01:50



Mathematics, 07.11.2020 01:50


Advanced Placement (AP), 07.11.2020 01:50

Social Studies, 07.11.2020 01:50