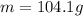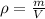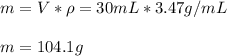Chemistry, 06.09.2020 06:01, victorialeverp714lg
If the density of a metal bar is 3.47 g/mL and volume is 30 mL, calculate the mass of the metal bar in grams (g). Show the steps to answering this equation.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:40, lindseyklewis1p56uvi
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization. a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution. part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 23.06.2019 02:30, babbity2009
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2

Chemistry, 23.06.2019 14:30, Izzyfizzy1
The hammering on a train track is often heard twice by workers farther down the track; first as the sound travels through the steel and second as the sound travels through the air. this suggests which graph is true?
Answers: 1
Do you know the correct answer?
If the density of a metal bar is 3.47 g/mL and volume is 30 mL, calculate the mass of the metal bar...
Questions in other subjects:



Biology, 14.10.2019 14:20


Mathematics, 14.10.2019 14:20

Mathematics, 14.10.2019 14:20