

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, hemolelekeakua
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 16:00, rorymartin04
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 23.06.2019 00:30, runninglovexoxo
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2
Do you know the correct answer?
An electron in the n = 3 energy level of the hydrogen atom emits a photon with wavelength 656.27 nm....
Questions in other subjects:


Mathematics, 12.03.2021 06:10



Mathematics, 12.03.2021 06:10



Mathematics, 12.03.2021 06:10

Mathematics, 12.03.2021 06:10

Mathematics, 12.03.2021 06:10


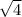 = 2
= 2



