
Chemistry, 06.09.2020 02:01, anthonybowie99
Calculate the mass of each of the following:
a. A sphere of gold with a radius of 10.5 cm. (The volume of a sphere with a radius r is V = (4/3)πr3; the density of gold is 19.3 g/cm^3.)
b. A cube of platinum of edge length 0.021 mm (density = 21.4 g/cm3).
c. 37.3 mL of ethanol (density = 0.798 g/mL).

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, mstrish71oteauw
A__ is two or more substances that are together in the same place but are not chemically combined
Answers: 1



Chemistry, 23.06.2019 03:00, rhianna18
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 1
Do you know the correct answer?
Calculate the mass of each of the following:
a. A sphere of gold with a radius of 10.5 cm. (The vol...
Questions in other subjects:











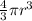
 = 4849.68 cm^3
= 4849.68 cm^3 =
= 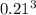 = 9.26 x 10^9 cm^3
= 9.26 x 10^9 cm^3



