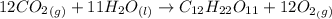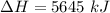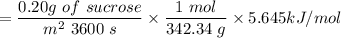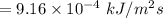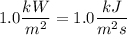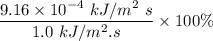Chemistry, 05.09.2020 01:01, joooselinn9688
The sun supplies about 1.0 kilowatt of energy for each square meter of surface area (1.0 kW/m^2 where a watt = 1 kJ/s) Plants produce the equivalent of about 0.20g of sucrose (C_12H_22O_11) per hour per square meter. Assuming that the sucrose is produced as follows, calculate the percentage of sunlight used to produce sucrose12CO2 (g) + 11H2O (I) --> C12H22O11 + 12O2(g) deltaH = 5645 kJ

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, johnsont8377
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1

Do you know the correct answer?
The sun supplies about 1.0 kilowatt of energy for each square meter of surface area (1.0 kW/m^2 wher...
Questions in other subjects:




History, 17.07.2019 08:00

Biology, 17.07.2019 08:00


Mathematics, 17.07.2019 08:00

English, 17.07.2019 08:00


Mathematics, 17.07.2019 08:00