Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, kylieweeks052704
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 09:00, kkmonsterhigh18
The diagram below shows a cell placed in a solution. a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution. only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it. it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 19:50, strawberrymrmr756
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

Chemistry, 22.06.2019 20:00, jalenevoyles
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3
Do you know the correct answer?
At a certain temperature, 0.338 mol CH and 0.831 mol HO is placed in a 3.50 L container.
CH(g)+2HO(...
Questions in other subjects:


Mathematics, 18.03.2021 01:40

Mathematics, 18.03.2021 01:40



Mathematics, 18.03.2021 01:40



Mathematics, 18.03.2021 01:40


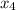 and 0.831 mol H
and 0.831 mol H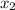 O is placed in a 3.50 L container.
CH
O is placed in a 3.50 L container.
CH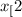 (g) At equilibrium, 6.51 g CO
(g) At equilibrium, 6.51 g CO