A solution is made by dissolving
37.5 g of sodium sulfide (Na2S) in
217 g of water.
Wha...

Chemistry, 03.09.2020 21:01, ilovecatsomuchlolol
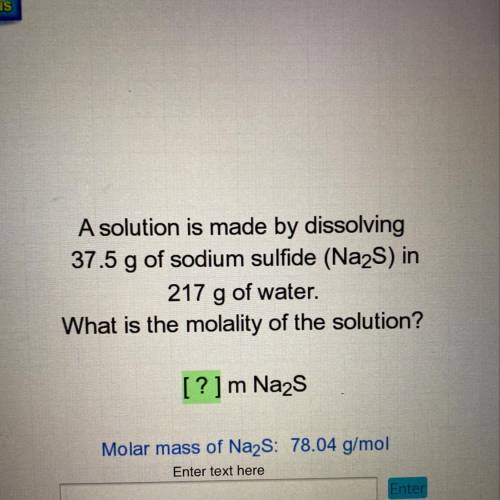
A solution is made by dissolving
37.5 g of sodium sulfide (Na2S) in
217 g of water.
What is the molality of the solution?

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:30, amylumey2005
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Advanced Placement (AP), 13.11.2019 07:31



English, 13.11.2019 07:31

Mathematics, 13.11.2019 07:31



English, 13.11.2019 07:31








