Chemistry, 02.09.2020 07:01, mohayon2020
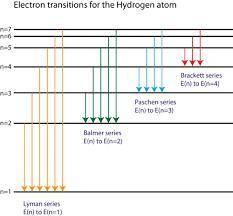
Calculate the wavelength of light produced if an electron moves from n=6 state to n=5 state of an electron in a hydrogen atom.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, david838843
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2

Chemistry, 22.06.2019 02:30, bionicboy03120440
What is the mass of sodium in 3 moles of sodium chloride
Answers: 1

Chemistry, 22.06.2019 08:00, PrincessKeliah5538
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 11:50, bellojamilet410
What substance has a mass of 9.5g and volume of 2.1cm^3
Answers: 2
Do you know the correct answer?
Calculate the wavelength of light produced if an electron moves from n=6 state to n=5 state of an el...
Questions in other subjects:

Computers and Technology, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01








Spanish, 23.09.2020 14:01


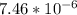 m
m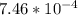 cm
cm