
Chemistry, 02.09.2020 22:01, shortyyashaun
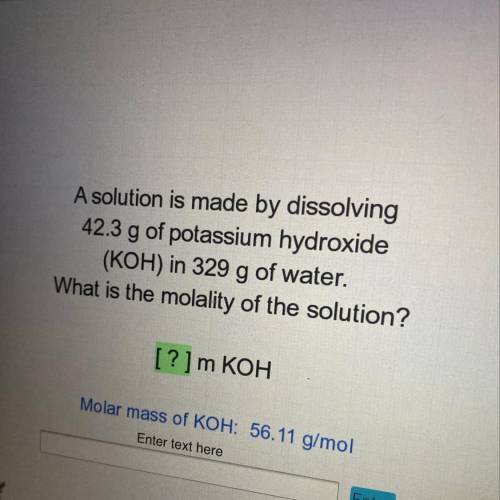
A solution is made by dissolving
42.3 g of potassium hydroxide
(KOH) in 329 g of water.
What is the molality of the solution?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 05:30, Dallas3506
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1


Chemistry, 23.06.2019 08:30, xojade
According to the passage, which of these is true about gray water systems? a) gray water systems use plants that require less water. eliminate b) gray water systems require the use of less fossil fuels. c) gray water systems reduce the amount of fresh water used. d) gray water systems reduce the amount water used by shower heads.
Answers: 1

Chemistry, 23.06.2019 10:30, aliyyahlove
Most ionic compouds are crystalline solids at room temperature. true falseionic compounds are electrically neutral. true falseionic compounds generally have low melting points. true falsewhen melted, ionic compounds do not conduct electricity. true falsethe electrostatic attraction between an anion and a cation is an ionic bond. true false
Answers: 1
Do you know the correct answer?
A solution is made by dissolving
42.3 g of potassium hydroxide
(KOH) in 329 g of water.
...
(KOH) in 329 g of water.
...
Questions in other subjects:

English, 29.01.2020 00:56



Business, 29.01.2020 00:56



Business, 29.01.2020 00:56

History, 29.01.2020 00:56








