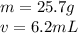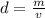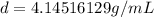Chemistry, 31.08.2020 07:01, homeworkprincess
An irregularly-shaped sample of aluminum (Al) is put on a balance and found to have a mass of 25.7 g. The student decides to use the water-displacement method to find the volume. The initial volume reading is 35.5 mL and, after the Al sample is added, the water level has risen to 41.7 mL. Find the density of the Al sample in g/mL. *

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, SilverTheAmarok
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 10:00, shayneseaton
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 14:10, cameronbeaugh
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 14:30, malenacastillo4887
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
Do you know the correct answer?
An irregularly-shaped sample of aluminum (Al) is put on a balance and found to have a mass of 25.7 g...
Questions in other subjects:


History, 02.12.2019 08:31





Mathematics, 02.12.2019 08:31


Chemistry, 02.12.2019 08:31

Mathematics, 02.12.2019 08:31



 is the mass and
is the mass and  is the volume.
is the volume.