Chemistry, 30.08.2020 20:01, lalala1212
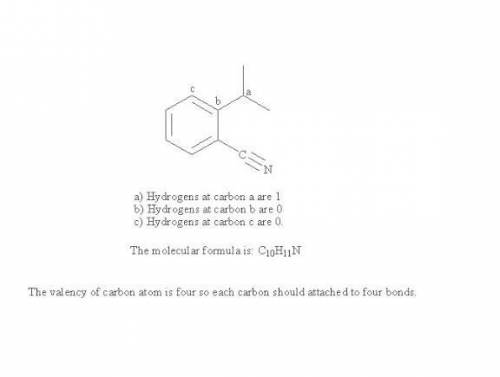
State the number of hydrogens bonded to each labeled carbon in the following substance and give its molecular formula. (The molecular formula answer is case-sensitive. The order of atoms should be carbon, then hydrogen, then others in alphabetical order.)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, coopera1744
Find the mass in grams of 1.37x1020 particles of h3po4
Answers: 2

Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 21:30, imalexiscv
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1
Do you know the correct answer?
State the number of hydrogens bonded to each labeled carbon in the following substance and give its...
Questions in other subjects:

Chemistry, 20.05.2021 22:30

Physics, 20.05.2021 22:30


Biology, 20.05.2021 22:30


Physics, 20.05.2021 22:30


Mathematics, 20.05.2021 22:30


Mathematics, 20.05.2021 22:30