
Chemistry, 31.08.2020 01:01, kleathers97
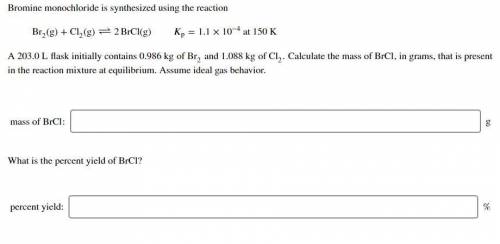
Bromine monochloride is synthesized using the reaction Br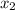 (g)+Cl
(g)+Cl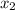 (g)↽−−⇀2BrCl(g) p=1.1×10
(g)↽−−⇀2BrCl(g) p=1.1×10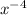 at 150 K A 203.0 L flask initially contains 0.986 kg of Br
at 150 K A 203.0 L flask initially contains 0.986 kg of Br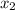 and 1.088 kg of Cl
and 1.088 kg of Cl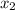 . Calculate the mass of BrCl , in grams, that is present in the reaction mixture at equilibrium. Assume ideal gas behavior. mass of BrCl : _g What is the percent yield of BrCl? percent yield: _%
. Calculate the mass of BrCl , in grams, that is present in the reaction mixture at equilibrium. Assume ideal gas behavior. mass of BrCl : _g What is the percent yield of BrCl? percent yield: _%

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, masteroftheuniverse3
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 13:30, xojade
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1


Chemistry, 23.06.2019 01:20, cedricevans41p4j3kx
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1
Do you know the correct answer?
Bromine monochloride is synthesized using the reaction Br(g)+Cl(g)↽−−⇀2BrCl(g) p=1.1×10 at 150 K A 2...
Questions in other subjects:

History, 17.09.2019 22:30


History, 17.09.2019 22:30


Mathematics, 17.09.2019 22:30




History, 17.09.2019 22:30







