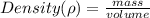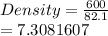Chemistry, 30.08.2020 02:01, alarconanais07
3. A block with a volume of 82.1 cm3 is made out of pure tin (Sn). If the mass of the block is 600. g, what is the density of the block?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, kajjumiaialome
Note the ph and poh values labeled with letters on the ph scale below. based on log rules and the way ph is calculated, what is the difference in [oh– ] concentration between point a and point b. a) 10^1 b) 10^5 c) 10^6 d) 10^7
Answers: 1

Chemistry, 22.06.2019 01:30, MickeyxX7096
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3


Chemistry, 22.06.2019 03:30, electrofy456
What diagram shows the ionic compound of magnesium oxide
Answers: 2
Do you know the correct answer?
3. A block with a volume of 82.1 cm3 is made out of pure tin (Sn). If the mass of the block is
600....
Questions in other subjects:


Mathematics, 14.01.2020 20:31


Mathematics, 14.01.2020 20:31




Social Studies, 14.01.2020 20:31

Physics, 14.01.2020 20:31

Mathematics, 14.01.2020 20:31