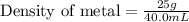Chemistry, 29.08.2020 05:01, jermainedwards
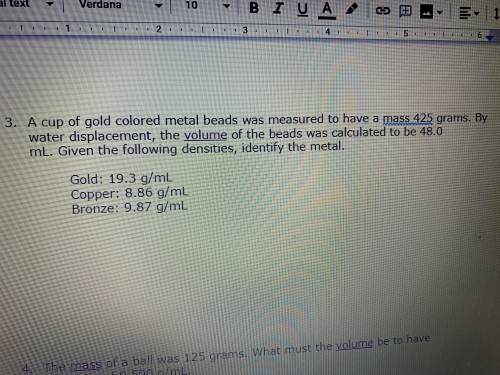
A cup of gold colored metal bed was measured to have a mask for 25 g. By water displacement, the volume of the bed was calculated to be 40.0 mL. Given the following densities, identify the metal. Gold equals 19.3 g/milliliters copper equals 8.86 g/milliliters bronze equals 9.87 g/milliliters

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:30, jabper5522
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 15:00, emmalie52
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
Do you know the correct answer?
A cup of gold colored metal bed was measured to have a mask for 25 g. By water displacement, the vol...
Questions in other subjects:

Mathematics, 16.02.2022 20:10



English, 16.02.2022 20:10



Biology, 16.02.2022 20:10

Mathematics, 16.02.2022 20:10