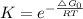Chemistry, 28.08.2020 16:01, j1theking18
Choose the best answer below. Which of the following reactions will have the largest equilibrium constant at 298 K?
a) 302(g) → 203(9) AGOrxn = +326 kJ
b) Mg(s) + N20(g) → Mgo(s) + N2(g) AG9rxn = -673.0 kJ
c) 2Hg(g) + O2(g) → 2HgO(s) AGºrx = -180.8 kJ
d) CaCO3(s) » Cao(s) + CO2(g) AG = +131.1 kJ
It is not possible to determine the reaction with the largest equilibrium constant using the given information.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:40, draveon353
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 01:30, montoyaricardo3550
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

Chemistry, 22.06.2019 11:40, arlabbe0606
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 16:00, yfnal3x
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
Do you know the correct answer?
Choose the best answer below. Which of the following reactions will have the largest equilibrium con...
Questions in other subjects:

Mathematics, 01.02.2020 13:42

Chemistry, 01.02.2020 13:42

Biology, 01.02.2020 13:42




Biology, 01.02.2020 13:42

Mathematics, 01.02.2020 13:42

Biology, 01.02.2020 13:42