
Chemistry, 25.08.2020 01:01, HalpMahOnMahH0meW0rk
In an experiment, 0.2 mol of copper atoms was dissolved in nitric acid to produce copper(ii)nitrate.
a)Calculate the volume of gas measured at r. t.p, produced in this reaction
b)calculatr the maximum mass of copper(ii)nitrate, produced in this reaction
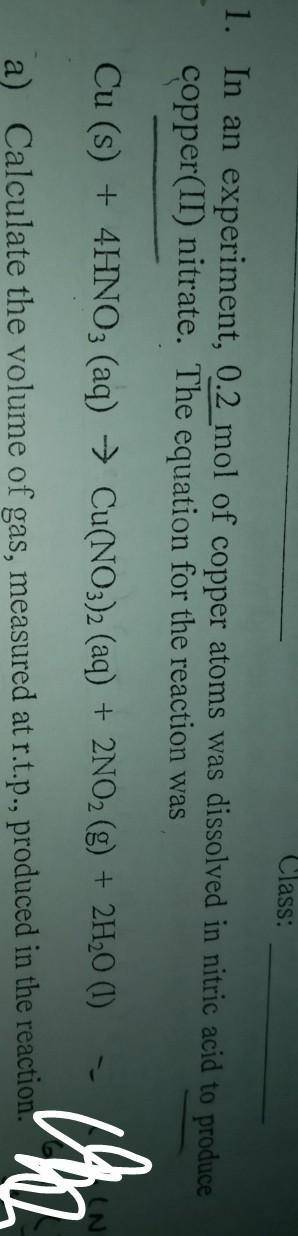

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, KnMcdonaldk93906
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 03:00, parisaidan366
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 11:50, robert7248
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3
Do you know the correct answer?
In an experiment, 0.2 mol of copper atoms was dissolved in nitric acid to produce copper(ii)nitrate....
Questions in other subjects:















