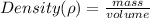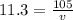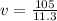Chemistry, 23.08.2020 20:01, pedroramirezr2
Lead has a density of 11.3 g/mL. If you have 105 grams of lead,
what is the volume in milliliters?
Remember to select an answer with the correct number of
significant figures.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:20, kingaman
Which of the following statements is not true? • a. covalent compounds have low melting and boiling points. • ob. covalent bonds between atoms of a compound are relatively weak compared to bonds between molecules. • c. covalent bonds occur between nonmetals. • d. covalent compounds are often gases or liquids.
Answers: 2

Chemistry, 22.06.2019 12:00, daytonalive83481
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1


Chemistry, 22.06.2019 18:10, bri9263
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
Do you know the correct answer?
Lead has a density of 11.3 g/mL. If you have 105 grams of lead,
what is the volume in milliliters?<...
Questions in other subjects:

History, 01.12.2020 22:40







Biology, 01.12.2020 22:40


Spanish, 01.12.2020 22:40