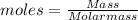Chemistry, 21.08.2020 14:01, pennyluvsu13
The molar mass of silver (Ag) is 107.87 g/mol.
Calculate the mass in grams of a sample of Ag containing 1.97 x 1022 atoms.
Write your answer using three significant figures.
g Ag

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, anglacx5465
Why are pipes bursting in the in extremely cold weather?
Answers: 2

Chemistry, 22.06.2019 10:10, dhailyortegacampa131
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 21:00, lucyamine0
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 22.06.2019 21:00, nsutton9985
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1
Do you know the correct answer?
The molar mass of silver (Ag) is 107.87 g/mol.
Calculate the mass in grams of a sample of Ag contai...
Questions in other subjects:

Mathematics, 19.11.2020 08:20

Mathematics, 19.11.2020 08:20



Mathematics, 19.11.2020 08:20

Health, 19.11.2020 08:20

Mathematics, 19.11.2020 08:20

English, 19.11.2020 08:20


Mathematics, 19.11.2020 08:20