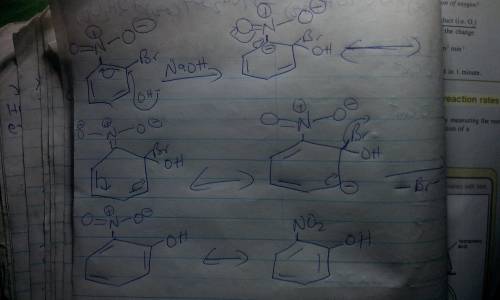
5. When ortho-bromonitrobenzene is treated with NaOH at elevated temperature, only one product is formed. (a) Draw the product. (b) Identify the intermediate formed en route to the product. (c) Would the reaction occur if the starting compound were meta-bromonitrobenzene

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 22:30, kristen17diaz
How many valence electrons are in atom of radon?
Answers: 1

Chemistry, 22.06.2019 22:30, xlebrny7831
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 22.06.2019 22:30, kiera2599
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1
Do you know the correct answer?
5. When ortho-bromonitrobenzene is treated with NaOH at elevated temperature, only one product is fo...
Questions in other subjects:



Mathematics, 07.05.2021 16:00

English, 07.05.2021 16:00

Mathematics, 07.05.2021 16:00

Arts, 07.05.2021 16:00

Mathematics, 07.05.2021 16:00

Mathematics, 07.05.2021 16:00


Mathematics, 07.05.2021 16:00