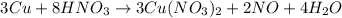Chemistry, 18.08.2020 15:01, laurabwhiddon
We explored the copper cycle. In the first step, copper was oxidized by nitric acid to make a green solution. Water was then added to the solution and the color changed from green to blue. Is this result supported by the spectrochemical series?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, PrincessKeliah5538
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 15:00, kamkam5791
Is powdered sports drink ionic or covalent ? 10pts !
Answers: 1

Chemistry, 23.06.2019 15:30, robert7248
K12 chemistry unit assessment: chemical bonding, electrostatic forces in ionic bonds hold which of the following together? 1. he atoms in helium gas 2.na+ and br- in nabr 3. fe atoms and localized electrons in iron
Answers: 1

Chemistry, 23.06.2019 18:30, tiwaribianca475
The graph shows one consequence of urban sprawl
Answers: 1
Do you know the correct answer?
We explored the copper cycle. In the first step, copper was oxidized by nitric acid to make a green...
Questions in other subjects:

Mathematics, 16.09.2019 14:30

History, 16.09.2019 14:30

Mathematics, 16.09.2019 14:30





Chemistry, 16.09.2019 14:30


Mathematics, 16.09.2019 14:30


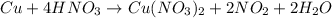
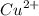 ) product which is initially coordinated to form nitrate ions from nitric acid and thus first gives the solution a green color.
) product which is initially coordinated to form nitrate ions from nitric acid and thus first gives the solution a green color.