Chemistry, 18.08.2020 22:01, allisongallion23
The standard free energy change for a reaction in an electrolytic cell is always:
a. Positive
b. Negative
c. Zero
d. Impossible to determine

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, jescanarias22
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1


Chemistry, 22.06.2019 16:00, yfnal3x
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 23.06.2019 15:00, jadentdaniels
Solve this problem using the appropriate law. (remember that ) what is the pressure of 1.9 mols of nitrogen gas in a 9.45 l tank and at a temperature of 228 k?
Answers: 1
Do you know the correct answer?
The standard free energy change for a reaction in an electrolytic cell is always:
a. Positive
Questions in other subjects:

Engineering, 29.08.2020 20:01



Mathematics, 29.08.2020 20:01






Mathematics, 29.08.2020 20:01


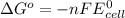
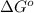 = standard free energy change
= standard free energy change 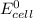 = standard emf
= standard emf