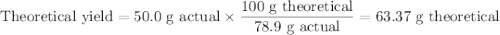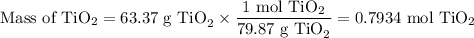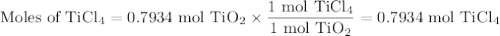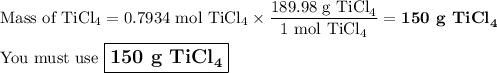Chemistry, 17.08.2020 23:01, leeahnnfoster
What mass of TiCl4 must react with an excess of water to produce 50.0g of TiO2 if the reaction has a 78.9% yield

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, caitybugking
Type the correct answer in the box. spell all words correctly. what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 12:30, quantamagic
Word equation for k(s)+h2o(l) yield koh(aq) + h2(g)
Answers: 1

Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 17:00, brownvester44
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2
Do you know the correct answer?
What mass of TiCl4 must react with an excess of water to produce 50.0g of TiO2 if the reaction has a...
Questions in other subjects:

Mathematics, 01.03.2021 05:20

Mathematics, 01.03.2021 05:20







Mathematics, 01.03.2021 05:20

Physics, 01.03.2021 05:20