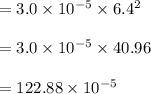Chemistry, 17.08.2020 01:01, nicholasferrell
The reaction NO2(g) + CO(g) → NO(g) + CO2(g) has been found to be second order with respect to NO2 and zero order with respect to CO. At a certain temperature, the rate constant is found experimentally to be 3.0 × 10−5 L mol · s . What is the rate of formation

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2


Chemistry, 22.06.2019 14:30, joejoefofana
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀ pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4. 0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 23.06.2019 11:30, melissalopez12
Place the following substances in order of ph from lowest ph to highest. a. neutral compounds, bases, acids b. acids, neutral compounds, bases c. bases, acids, neutral compounds d. bases, neutral compounds, acids
Answers: 1
Do you know the correct answer?
The reaction NO2(g) + CO(g) → NO(g) + CO2(g) has been found to be second order with respect to NO2 a...
Questions in other subjects:

Geography, 23.10.2021 06:10

Social Studies, 23.10.2021 06:10

Mathematics, 23.10.2021 06:10

Mathematics, 23.10.2021 06:20




Mathematics, 23.10.2021 06:20


Social Studies, 23.10.2021 06:20


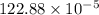 "
"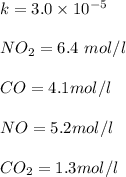

![=k.[NO_2]^2](/tpl/images/0723/2177/ff6c8.png) because the above given is the part of the second-order, which relates to
because the above given is the part of the second-order, which relates to 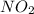 . In the zeros order the Carbon monoxide (CO) its reaction doesn't affect the rate.
. In the zeros order the Carbon monoxide (CO) its reaction doesn't affect the rate.