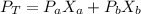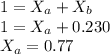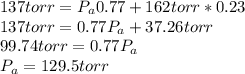Chemistry, 16.08.2020 14:01, ant5784tgi
Two volatile substances, A and B are dissolved in one another and the resulting solution has a vapor pressure of 137 torr. If the mole fraction of B is 0.230 and the vapor pressure of pure B is 162 torr, what is the vapor pressure of pure A in torr?

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 17:30, shookiegriffin
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

Chemistry, 23.06.2019 08:40, mathisaqeosmw
Calculate the number of grams of sodium in 3.00 g of each sodium-containing food additive.
Answers: 3
Do you know the correct answer?
Two volatile substances, A and B are dissolved in one another and the resulting solution has a vapor...
Questions in other subjects:

Computers and Technology, 07.06.2021 05:30





Mathematics, 07.06.2021 05:30



Mathematics, 07.06.2021 05:30

Mathematics, 07.06.2021 05:30