
Chemistry, 15.08.2020 19:01, mivantechenko9751
A solution is prepared by adding 0.0231moles of H3O+ ions to 3.33L of water. What is the pH of this solution

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 15:30, dhernandez081
Acontainer holds 6.4 moles of gas. hydrogen gas makes up 25% of the total moles in the container. if the total pressure is 1.24atm. what is the partial pressure of hydrogen
Answers: 3

Chemistry, 23.06.2019 16:00, paigejosie6473
What three relationships are true at equilibrium
Answers: 1

Chemistry, 24.06.2019 00:40, sugar1014
The complete combustion of octane, a component of gasoline, is represented by the equation: 2 c8h18(l) + 25 o2(g) →16 co2(g) + 18 h2o(l) how many liters of co2(g), measured at 63.1°c and 688 mmhg, are produced for every gallon of octane burned? (1 gal = 3.785 l; density of c8h18(l) = 0.703 g/ml)
Answers: 1
Do you know the correct answer?
A solution is prepared by adding 0.0231moles of H3O+ ions to 3.33L of water. What is the pH of this...
Questions in other subjects:


Biology, 16.04.2021 01:00

Mathematics, 16.04.2021 01:00


Mathematics, 16.04.2021 01:00



Mathematics, 16.04.2021 01:00



![pH~=~-Log[H_3O^+]](/tpl/images/0722/7168/29200.png)
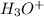 ). So, the next step is the calculation of the concentration of the hydronium ions. For this, we have to use the molarity formula:
). So, the next step is the calculation of the concentration of the hydronium ions. For this, we have to use the molarity formula: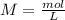
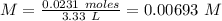
![pH~=~-Log[0.00693~M]~=~2.15](/tpl/images/0722/7168/1523c.png)




