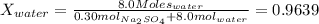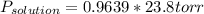Chemistry, 15.08.2020 01:01, nathanwhite2000
at 25*C, the vapor pressure of pure water is 23.8 torr. a solution is prepared by dissolving 0.30 mol Na2SO4 in 8.0 moles of water. what is the vapor pressure of this solution at 25*C

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, PSBSolarYT
For which one of the following reactions is the value of δh° rxn equal to δh° f for the product? a. 2 h2 (g) + o2 (g) → 2 h2o (l) b. n2 (g) + o2 (g) → 2 no (g) c. 2 h2 (g) + o2 (g) → 2 h2o (g) d. h2o (l) + 1/2 o2 (g) → h2o2 (l) e. none of the above
Answers: 1

Chemistry, 21.06.2019 18:30, brookekolmetz
How many orbitals does the p sub shell container
Answers: 3


Chemistry, 22.06.2019 19:00, georgesarkes12
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
Do you know the correct answer?
at 25*C, the vapor pressure of pure water is 23.8 torr. a solution is prepared by dissolving 0.30 mo...
Questions in other subjects:




Mathematics, 15.04.2020 02:46

Mathematics, 15.04.2020 02:46