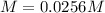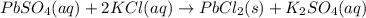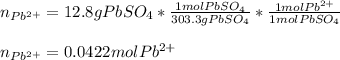Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, hala201490
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1


Chemistry, 23.06.2019 06:10, tammydbrooks43
How much would the freezing point of water decrease if 4 mol of nacl were added to 1 kg of water (kf=1.86 degrees c/(mol/kg) for water and i=2 for nacl a- 7.44 degrees c b- 14.88 c 3.72 d 1.86
Answers: 1

Chemistry, 23.06.2019 07:00, tiarafaimealelei
Select the correct answer. why are scientific models important in the study of science? a. they always involve critical mathematical calculations. b. they scientists understand complex ideas and objects that aren’t easy to handle. c. they enable scientists to popularize their work in society. d. they are required when conducting any peer review process. e. they are necessary for turning a hypothesis into a law.
Answers: 2
Do you know the correct answer?
If 12.8 g lead(II) sulfate (303.3 g/mol) precipitates when excess potassium chloride is added to 1.6...
Questions in other subjects:


Mathematics, 05.02.2021 06:30

Social Studies, 05.02.2021 06:30

Mathematics, 05.02.2021 06:30


Chemistry, 05.02.2021 06:30

Mathematics, 05.02.2021 06:30