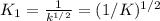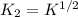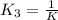Chemistry, 14.08.2020 05:01, ramireznaidelyn
Methanol liquid burns readily in air. One way to represent this equilibrium is: 2 CO2(g) + 4 H2O(g)2 CH3OH(l) + 3 O2(g) We could also write this reaction three other ways, listed below. The equilibrium constants for all of the reactions are related. Write the equilibrium constant for each new reaction in terms of K, the equilibrium constant for the reaction above. 1) CH3OH(l) + 3/2 O2(g) CO2(g) + 2 H2O(g) K1 = 2) CO2(g) + 2 H2O(g) CH3OH(l) + 3/2 O2(g) K2 = 3) 2 CH3OH(l) + 3 O2(g) 2 CO2(g) + 4 H2O(g)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, parisaidan366
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3


Chemistry, 22.06.2019 19:30, amandamiro05
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 19:30, dorindaramirez0531
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1
Do you know the correct answer?
Methanol liquid burns readily in air. One way to represent this equilibrium is: 2 CO2(g) + 4 H2O(g)2...
Questions in other subjects:


Mathematics, 30.08.2019 07:10





History, 30.08.2019 07:10



Mathematics, 30.08.2019 07:10