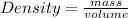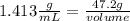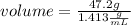Chemistry, 14.08.2020 01:01, reginaldboyd28
The density of concentrated nitric acid (HNO3) is 1.413 g/mL. What volume in liters would be occupied by a mass of 47.2 g?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, nekathadon
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1


Chemistry, 23.06.2019 13:30, medellincolombia99
Which of these statements describes the size of an atom? a. an atom is larger than a sheet of aluminum foil. b. an atom is small but can be seen with just our eyes. c. an atom is the size of a plastic building block. d. an atom is tiny and cannot be seen without magnification.
Answers: 2
Do you know the correct answer?
The density of concentrated nitric acid (HNO3) is 1.413 g/mL. What volume in liters would be occupie...
Questions in other subjects:

Geography, 11.05.2021 18:40

Chemistry, 11.05.2021 18:40




Social Studies, 11.05.2021 18:40




Spanish, 11.05.2021 18:40