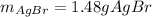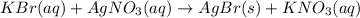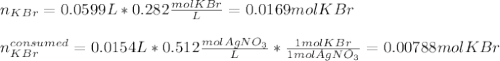Chemistry, 14.08.2020 01:01, jakobrobinette
g A chemist combines 59.9 mL of 0.282 M potassium bromide with 15.4 mL of 0.512 M silver nitrate. (a) How many grams of silver bromide will precipitate

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, salvadorperez26
Asolution has a ca2+ concentration of 0.049 m and an f- concentration is 0.147 m at equilibrium. the value of ksp for caf2 at 25°c is 4.0 x 10-11. will this solution form a precipitate? yes no
Answers: 3

Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 21:00, andrethisman88
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 22.06.2019 21:30, emmalucilleblaha1995
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
Do you know the correct answer?
g A chemist combines 59.9 mL of 0.282 M potassium bromide with 15.4 mL of 0.512 M silver nitrate. (a...
Questions in other subjects:



Chemistry, 20.11.2021 05:00






Chemistry, 20.11.2021 05:00

Mathematics, 20.11.2021 05:00