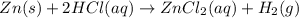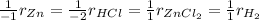Chemistry, 13.08.2020 01:01, ayoismeisalex
In the reaction of Zn(s) + 2HCl (aq) Imported Asset ZnCl2 (aq) + H2 (g), if [HCl] increases from 2.6 M to 8.2 M:
The rate at which Zn disappears decreases.
The rate at which H2 appears decreases.
The rate at which ZnCl2 appears increases.
The concentration of Zn (s) also increases.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:20, icantspeakengles
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2


Chemistry, 24.06.2019 01:30, alishakira690
Which of these is most likely a characteristic of a non-radioactive daughter isotope after it is formed from the parent isotope? 1) stable electrons 2) stable nucleus 3) positively charged atom 4) negatively charged atom
Answers: 1

Chemistry, 24.06.2019 01:40, Ashtree519
And someone plz me with questions 5 and 6 plz this is for masteries
Answers: 2
Do you know the correct answer?
In the reaction of Zn(s) + 2HCl (aq) Imported Asset ZnCl2 (aq) + H2 (g), if [HCl] increases from 2.6...
Questions in other subjects:

Mathematics, 07.12.2021 17:50


History, 07.12.2021 17:50

Mathematics, 07.12.2021 17:50




Mathematics, 07.12.2021 17:50

Mathematics, 07.12.2021 17:50

Mathematics, 07.12.2021 17:50