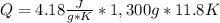Chemistry, 12.08.2020 08:01, runninglovexoxo
Calculate the energy required to heat 1.30kg of water from 22.4°C to 34.2°C . Assume the specific heat capacity of water under these conditions is 4.18·J·g−1K−1 . Round your answer to 3 significant digits.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:10, dhailyortegacampa131
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 15:00, MilanPatel
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Do you know the correct answer?
Calculate the energy required to heat 1.30kg of water from 22.4°C to 34.2°C . Assume the specific he...
Questions in other subjects:



Chemistry, 15.01.2020 19:31

Biology, 15.01.2020 19:31


Mathematics, 15.01.2020 19:31


Mathematics, 15.01.2020 19:31

Mathematics, 15.01.2020 19:31

History, 15.01.2020 19:31


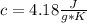 m= 1.30 kg= 1,300 g (1 kg=1,000 g)ΔT= 34.2 °C - 22.4 °C= 11.8 °C= 11.8 °K Being a temperature difference, it is independent if they are degrees Celsius or degrees Kelvin. That is, the temperature difference is the same in degrees Celsius or degrees Kelvin.
m= 1.30 kg= 1,300 g (1 kg=1,000 g)ΔT= 34.2 °C - 22.4 °C= 11.8 °C= 11.8 °K Being a temperature difference, it is independent if they are degrees Celsius or degrees Kelvin. That is, the temperature difference is the same in degrees Celsius or degrees Kelvin.