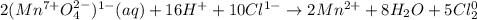Chemistry, 12.08.2020 08:01, Kaziyah461
After balancing the following reaction under acidic conditions, how many mole equivalents of water are required and on which side of the reaction do they appear?
MnO41- (aq) + Cl1- (aq) → Mn2+ (aq) + Cl2 (g)
a. 2 moles of H2O on the reactant side
b. 2 moles of H2O on the product side
c. 4 moles of H2O on the product side
d. 8 moles of H2O on the product side
e. 10 moles of H2O on the reactant side

Answers: 3
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 00:30, terryg4397
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
Do you know the correct answer?
After balancing the following reaction under acidic conditions, how many mole equivalents of water a...
Questions in other subjects:



History, 01.12.2021 22:40

Physics, 01.12.2021 22:40



Mathematics, 01.12.2021 22:40


Arts, 01.12.2021 22:40

Biology, 01.12.2021 22:40


![MnO_4^{1-} (aq) + Cl^{1-} (aq) \rightarrow Mn^{2+} (aq) + Cl_2 (g)\\\\(Mn^{7+}O^{2-}_4)^{1-} (aq) + Cl^{1-} (aq) \rightarrow Mn^{2+} (aq) + Cl_2 (g)\\\\\\\\(Mn^{7+}O^{2-}_4)^{1-} (aq)+8H^++5e^- \rightarrow Mn^{2+}+4H_2O\\\\2Cl^{1-}\rightarrow Cl_2^0+2e^-\\\\2*[(Mn^{7+}O^{2-}_4)^{1-} (aq)+8H^++5e^- \rightarrow Mn^{2+}+4H_2O]\\\\5*[2Cl^{1-}\rightarrow Cl_2^0+2e^-]\\\\\\\\2(Mn^{7+}O^{2-}_4)^{1-} (aq)+16H^++10e^- \rightarrow 2Mn^{2+}+8H_2O\\\\10Cl^{1-}\rightarrow 5Cl_2^0+10e^-\\](/tpl/images/0720/0206/c85d6.png)