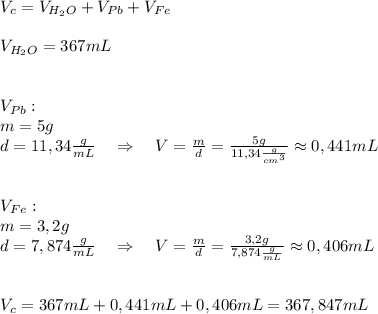A5.0 gram sample of lead and a 3.2 g sample of iron are placed into 367 ml of water. what will be the new volume level of water in units of ml? the density of lead is 11.34 g/cc and the density of iron is 7.874 g/ml. round your answer to three significant figures

Answers: 1
Similar questions

Chemistry, 09.07.2019 11:10, klrm8045
Answers: 1



Chemistry, 18.10.2019 09:30, haru8355
Answers: 1
Do you know the correct answer?
A5.0 gram sample of lead and a 3.2 g sample of iron are placed into 367 ml of water. what will be th...
Questions in other subjects:

SAT, 30.07.2021 01:00


Mathematics, 30.07.2021 01:00

Mathematics, 30.07.2021 01:00