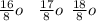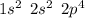. A certain diatomic element is a colorless gas and is highly reactive. It has three isotopes with mass #’s 16, 17 and 18 i. What is the naturally occurring diatomic element ? (2 points) ii. Write the isotopic symbols. (2 points) iii. How many neutrons does each possess? (2 points) iv. What is the electronic configuration of each isotope?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, tahjaybenloss16
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 23.06.2019 01:30, Nathaliasmiles
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2

Do you know the correct answer?
. A certain diatomic element is a colorless gas and is highly reactive. It has three isotopes with m...
Questions in other subjects:

History, 28.06.2019 23:30

Chemistry, 28.06.2019 23:30

Biology, 28.06.2019 23:30



Chemistry, 28.06.2019 23:30

Mathematics, 28.06.2019 23:30

Mathematics, 28.06.2019 23:30


Mathematics, 28.06.2019 23:30