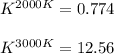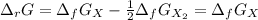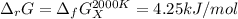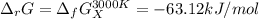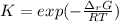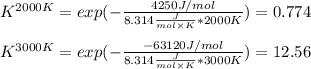Chemistry, 12.08.2020 04:01, anitadefrances
The equation represents the decomposition of a generic diatomic element in its standard state. 12X2(g)⟶X(g) Assume that the standard molar Gibbs energy of formation of X(g) is 4.25 kJ·mol−1 at 2000. K and −63.12 kJ·mol−1 at 3000. K. Determine the value of K (the thermodynamic equilibrium constant) at each temperature.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, mazielynn84
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 10:40, rntaran2002
What is the ph of a 0.0010 m hno3? 1.0 3.0 4.0 5.0
Answers: 2

Chemistry, 23.06.2019 03:30, HalpMahOnMahH0meW0rk
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1

Chemistry, 23.06.2019 10:30, staceymrtosr
Amethod of separation that employs a system with two phases of matter, a mobile phase and a stationary phase, is called
Answers: 2
Do you know the correct answer?
The equation represents the decomposition of a generic diatomic element in its standard state. 12X2(...
Questions in other subjects:

Social Studies, 13.04.2021 01:10




Mathematics, 13.04.2021 01:10

Mathematics, 13.04.2021 01:10



Mathematics, 13.04.2021 01:10