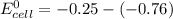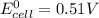Chemistry, 12.08.2020 04:01, kingjames82
Using these metal ion/metal standard reduction potentials Cd2+(aq)|Cd(s) Zn2+(aq)|Zn(s) Ni2+(aq)|Ni(s) Cu2+(aq)/Cu(s) -0.40 V -0.76 V ‑0.25 V +0.34 V Calculate the standard cell potential for the cell whose reaction is Ni2+(aq) + Zn(s) →Zn2+(aq)+ Ni(s)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:40, nadinealonzo6121
Which of the following might a chemist choose to study? a. glacier movement in alaska b. better ways to recycle plastics c. the effects of hurricanes on turtle populations d. the vibrations in bridges caused by big trucks
Answers: 2


Chemistry, 22.06.2019 02:10, fvmousdiana
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 14:30, davidrodriguez122001
Which of the following describes a situation where competition between producers exists
Answers: 1
Do you know the correct answer?
Using these metal ion/metal standard reduction potentials Cd2+(aq)|Cd(s) Zn2+(aq)|Zn(s) Ni2+(aq)|Ni(...
Questions in other subjects:


English, 24.09.2021 14:40

Social Studies, 24.09.2021 14:40

Mathematics, 24.09.2021 14:40



Mathematics, 24.09.2021 14:40



Mathematics, 24.09.2021 14:40


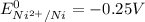
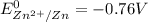
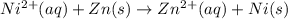
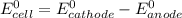
 are standard reduction potentials.
are standard reduction potentials.