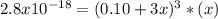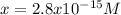Chemistry, 12.08.2020 06:01, shanilafaridor97hl
The solubility product for Ag3PO4 is 2.8 × 10‑18. What is the solubility of silver phosphate in a solution which also contains 0.10 moles of silver nitrate per liter?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, erinxmeow8
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons, neutrons, electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 08:30, mosthatedpicky1
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 09:00, tbiles99
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1
Do you know the correct answer?
The solubility product for Ag3PO4 is 2.8 × 10‑18. What is the solubility of silver phosphate in a so...
Questions in other subjects:




History, 07.11.2019 03:31



History, 07.11.2019 03:31


Mathematics, 07.11.2019 03:31


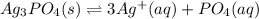
![Ksp=[Ag^+]^3[PO_4^-]](/tpl/images/0718/8186/a4d33.png)
 :
: