
Chemistry, 12.08.2020 06:01, kayleg907436
In the buffer solution image Question 1 options: A) CH3CO2H is a base, and H3O+ is its conjugate acid. B) H3O+ is an acid, and CH3CO2 – is its conjugate base. C) CH3CO2H is an acid, and CH3CO2 – is its conjugate base. D) H3O+ is an acid, and CH3CO2H is its conjugate base.
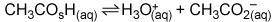

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:00, alanmarcus22
What does the symbol (–hfus) indicate in a phase change?
Answers: 1

Chemistry, 23.06.2019 00:00, alisonsolis155
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3
Do you know the correct answer?
In the buffer solution image Question 1 options: A) CH3CO2H is a base, and H3O+ is its conjugate aci...
Questions in other subjects:


Mathematics, 25.06.2019 16:00

Geography, 25.06.2019 16:00

History, 25.06.2019 16:00


English, 25.06.2019 16:00


Mathematics, 25.06.2019 16:00







