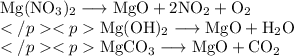Chemistry, 05.08.2020 15:01, blackeyes7659
Magnesium nitrate, magnesium hydroxide and magnesium carbonate all decompose when heated.
Which statement about these decomposition reactions is correct?
A. Magnesium carbonate decomposes to release carbon dioxide and oxygen
B. Magnesium hydroxide decomposes to release hydrogen and oxygen
C. Magnesium hydroxide decomposes to release water vapour
D. Magnesium nitrate decomposes to release oxygen only

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, Cythina2007
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 10:00, emfranco1
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 19:00, miguel454545
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 20:30, lexibyrd120
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2
Do you know the correct answer?
Magnesium nitrate, magnesium hydroxide and magnesium carbonate all decompose when heated.
Which sta...
Questions in other subjects:


Mathematics, 30.05.2020 05:57





Mathematics, 30.05.2020 05:58


History, 30.05.2020 05:58