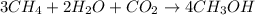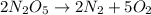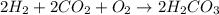Chemistry, 12.08.2020 06:01, rosanaboyd7
Which of the following is the balanced reaction, given the rate relationships below.
a. rate = − 13 Δ[CH4] Δt = − 12 Δ[H2O] Δt = − Δ[CO2] Δt = 14 Δ[CH3OH] Δt
b. rate = − 12 Δ[N2O5] Δt = 12 Δ[N2] Δt = 15 Δ[O2] Δt
c. rate = − 12 Δ[H2] Δt = − 12 Δ[CO2] Δt = − Δ[O2] Δt = 12 Δ[H2CO3] Δt

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, awdadaddda
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 22.06.2019 06:30, dimondqueen511
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 10:00, berniceallonce22
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 18:30, tanviknawale
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
Do you know the correct answer?
Which of the following is the balanced reaction, given the rate relationships below.
a. rate = − 13...
Questions in other subjects:

Mathematics, 15.09.2020 21:01

Mathematics, 15.09.2020 21:01

Mathematics, 15.09.2020 21:01

Mathematics, 15.09.2020 21:01

Mathematics, 15.09.2020 21:01

Mathematics, 15.09.2020 21:01

Mathematics, 15.09.2020 21:01

Mathematics, 15.09.2020 21:01

Mathematics, 15.09.2020 21:01

Mathematics, 15.09.2020 21:01