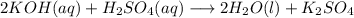Chemistry, 12.08.2020 06:01, kaylaelaine18
What is the complete ionic equation for this reaction?
2KOH(aq) + H2SO4(aq) → 2H20(1) + K2SO4(aq)
O A. 2K+ + OH + H2SO4 → OH + 2H+ + K2SO4
B. OH + 2H+ + 2H20()
C. 2KOH + H2SO4 → 2H20 + K2SO4
D. 2K+ + OH + 2H+ + SO42- → 2H20() + 2K+ + SO42-
SUBMIT

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, Riplilpeep
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1



Chemistry, 22.06.2019 20:00, rafaelasoareschagas7
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3
Do you know the correct answer?
What is the complete ionic equation for this reaction?
2KOH(aq) + H2SO4(aq) → 2H20(1) + K2SO4(aq)
Questions in other subjects:


Mathematics, 30.08.2019 00:30







History, 30.08.2019 00:30

Mathematics, 30.08.2019 00:30