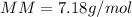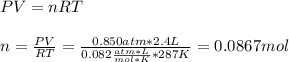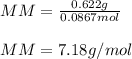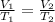Chemistry, 04.08.2020 23:01, jamarengle2
Part 1. Determine the molar mass of a 0.622-gram sample of gas having a volume of 2.4 L at 287 K and 0.850 atm. Show your work. Part 2. If this sample was placed under extremely low temperature, describe how the actual volume would compare to the predicted volume. Explain your answer.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:30, jewelz5887
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 14:30, Cartucho1978
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 16:00, anferneebcoleman
How many moles of oxygen react with 12 moles of aluminum
Answers: 1
Do you know the correct answer?
Part 1. Determine the molar mass of a 0.622-gram sample of gas having a volume of 2.4 L at 287 K and...
Questions in other subjects:


Social Studies, 23.10.2019 05:10

History, 23.10.2019 05:10


Geography, 23.10.2019 05:10

Mathematics, 23.10.2019 05:10

Mathematics, 23.10.2019 05:10

Chemistry, 23.10.2019 05:10

Mathematics, 23.10.2019 05:10

English, 23.10.2019 05:10