
Chemistry, 04.08.2020 23:01, elijahjacksonrp6z2o7
Part 1. Determine the molar mass of a 0.622-gram sample of gas having a volume of 2.4 L at 287 K and 0.850 atm. Show your work. Part 2. If this sample was placed under extremely low temperature, describe how the actual volume would compare to the predicted volume. Explain your answer.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, lindseyklewis1p56uvi
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 23.06.2019 00:00, alisonsolis155
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3

Chemistry, 23.06.2019 10:50, uh8hardiek
Mach the labels with the symbols on the weather map
Answers: 2

Chemistry, 23.06.2019 11:50, vmosley8648
It takes 155. kj/mol to break a fluorine-fluorine single bond. calculate the maximum wavelength of light for which a flouine-flouring single bond could be broken by absorbing a single photon
Answers: 1
Do you know the correct answer?
Part 1. Determine the molar mass of a 0.622-gram sample of gas having a volume of 2.4 L at 287 K and...
Questions in other subjects:


Spanish, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01


Mathematics, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01

Law, 16.10.2020 18:01



Mathematics, 16.10.2020 18:01


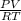
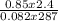
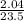 = 0.087mole
= 0.087mole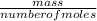
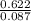 =7.15gmol⁻¹
=7.15gmol⁻¹



