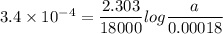Chemistry, 03.08.2020 14:01, lenniestreet10
For a first-order reaction, A → B, the rate coefficient was found to be 3.4 × 10-4 s-1 at 23 °C. After 5.0 h, the concentration of A was found to be 0.00018 mol L-1. What was the original concentration of A?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, lasagnafoe
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2


Chemistry, 22.06.2019 12:30, hayleyconsole
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3
Do you know the correct answer?
For a first-order reaction, A → B, the rate coefficient was found to be 3.4 × 10-4 s-1 at 23 °C. Aft...
Questions in other subjects:


Physics, 25.11.2021 08:50


History, 25.11.2021 08:50

Chemistry, 25.11.2021 08:50

Mathematics, 25.11.2021 08:50




History, 25.11.2021 08:50